-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
A Standardized Boswellia serrata Extract Improves Knee Joint Function and Cartilage Morphology in Human Volunteers with Mild to Moderate Osteoarthritis in a Randomized Placebo-Controlled Study

A Standardized Boswellia serrata Extract Improves Knee Joint Function and Cartilage Morphology in Human Volunteers with Mild to Moderate Osteoarthritis in a Randomized Placebo-Controlled Study
Brijesh Kumar, Abhijeet Balbhim Ghaytidak, Abhinav Kumar Pandey, Raghu Ram Somepalli, Praveen Sarda, Siba Prasad Raychaudhuri & Meher Prasanna Rokkam (19 Dec 2024): A Standardized Boswellia serrata Extract Improves Knee Joint Function and Cartilage Morphology in Human Volunteers with Mild to Moderate Osteoarthritis in a Randomized Placebo-Controlled Study, Journal of the American Nutrition Association, DOI: 10.1080/27697061.2024.2438894
Background and objective: Boswellia serrata Roxb. ex Colebr. (Family: Burseraceae; Genus: Boswellia) gum resin (Salai guggul) has profound therapeutic value in Ayurvedic and Unani medicines in alleviating several chronic inflammatory illnesses, including arthritis, asthma, skin and blood diseases, fever, etc. SN13108F (AprèsFlex®) is a proprietary, standardized Boswellia serrata gum resin extract. This 180-day randomized, placebo-controlled clinical study aimed to evaluate cartilage morphology using magnetic resonance imaging (MRI), pain and joint function and long-term safety in the SN13108F-supplemented volunteers with knee osteoarthritis (KOA).
Materials and methods: Eighty adult male and female subjects with the Kellgren-Lawrence grade II - III KOA were supplemented with SN13108F (100 mg/day) or a matched placebo for 180 consecutive days.
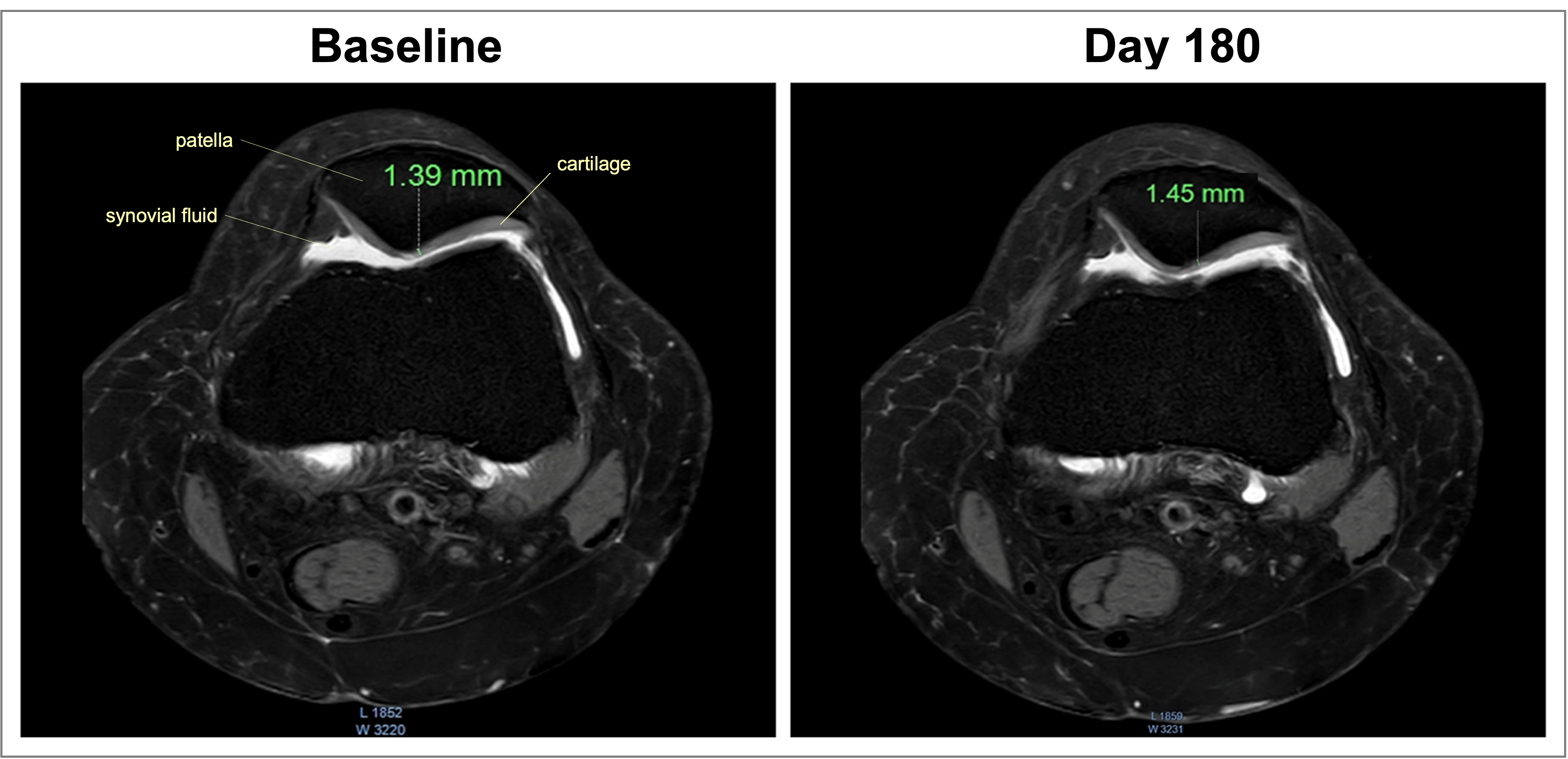
Results: SN13108F reduced (p < 0.001; vs. baseline and placebo) Western Ontario and McMaster Universities Osteoarthritis Index, Visual Analogue Scale, Lequesne’s Functional Index scores, improved six-minute walk test, and stair climb test. Post-trial MRI assessments of the tibiofemoral joints revealed that the cartilage volume, thickness, and joint space width were increased (p < 0.001; vs. placebo), and levels of high-sensitivity C-reactive protein, matrix metalloproteinase-3, Fibulin-3, type II collagen degradation peptide in serum, and cross-linked C-terminal telopeptide of type II collagen in urine were significantly reduced (p < 0.001; vs. baseline and placebo) in the SN13108F-supplemented subjects. Hematology, complete serum biochemistry, urine analysis, and the participants’ vital signs did not alter between the groups.
Conclusion: SN13108F supplementation is safe, and it mitigates joint pain and improves musculoskeletal function and cartilage morphology in KOA.














