-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
AprèsFlex® 5-Day Joint Support

OVERVIEW
AprèsFlex® is one of the world’s best-selling joint health ingredients – with multiple clinical studies that demonstrate efficacy starting at 5 days – with a low dose.

5 Days
AprèsFlex has been shown in clinical trials to improve joint comfort in as little as 5 days.

100mg
Proven efficacy at only 100mg/day.

11 Studies
The science behind AprèsFlex is solid. It is supported by 7 preclinical and 4 human clinical trials.

6 Months
AprèsFlex has been clinically shown to continuously improve joint comfort and function at six months.
FEATURES & BENEFITS
Low, 100mg/day dose |
|
Fast acting joint comfort in just 5 days* |
|
56% reduction in WOMAC pain scores at 30 days* |
|
70% reduction in WOMAC pain scores at 6 months* |
|
Improved knee joint space and reduction of cartilage degradation* |
|
Preservation of cartilage and improved cartilage health* |
|
Four double-blind clinical trials |
|
Sustainable, botanical ingredient |

CERTIFICATIONS
VEGAN
GLUTEN-FREE
NON-GMO
HALAL
KOSHER
A Patented, Synergistic Extract of Boswellia serrata
In joint health products, AprèsFlex is powering some of the best-known joint health consumer brands in the world.
Boswellia serrata
Sourced from the forests of India, Boswellia has been used in Ayurvedic medicine for thousands of years.

A Sacred Tree
Boswellia serrata are considered sacred by Indian families and given as gifts at weddings.
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Detail 1
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.

Detail 2
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.
RESEARCH
One of the most clinically studied mobility support ingredients on the market
AprèsFlex 5-Day Joint Support has been the subject of extensive preclinical and clinical research to demonstrate its mechanisms of action and efficacy in supporting joint comfort and mobility.
In its third clinical study, AprèsFlex was found to:
• Provide significant reduction (p<0.05) in all pain scores at five days compared to the placebo*
• Provide significant improvement (p<0.05) in all function scores at five days compared to placebo*
Visual Analog Score (VAS) (Graph A)
Lequesne Index (LFI) (Graph B)
WOMAC Index ((Graphs C, D, E)
Long-Term Performance
In its fourth clinical trial, at six months, AprèsFlex was found to:
• Improve joint comfort by 70%*
• Reduce stiffness by 72%*
• Enhance physical function*
• Improve walking speed by 27%*
Cartilage Preservation and Protection
In its fourth clinical trial, AprèsFlex was found to:
• Mitigate loss of joint space compared to placebo*
• Preserve cartilage thickness*
• Reduce markers of cartilage breakdown*
(^p<0.05 vs placebo)
For more information on AprèsFlex science, see the Resources section below.
Groundbreaking MRI Analysis
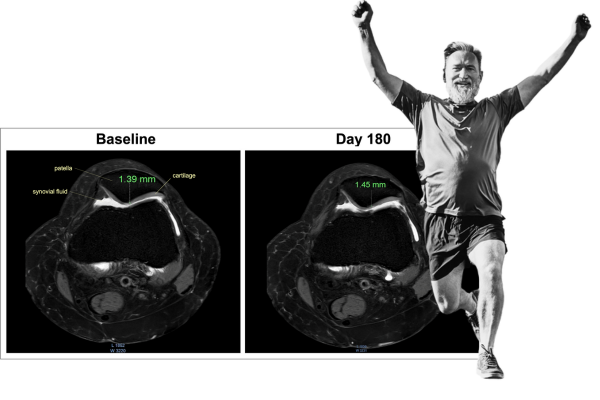
The clinical study used MRI to measure the impact of AprèsFlex on cartilage health. MRI analyses revealed that 180 days of AprèsFlex supplementation improved the participants’ knee joint space width, cartilage thickness, and volume. Below are representative MRI Images of one knee joint showing joint space width and femoral, medial, lateral, and patellar cartilage thickness and volume captured at baseline and on day 180 of AprèsFlex supplementation. Post-trial, the intergroup comparison analysis revealed that the improvements (from baseline) in the AprèsFlex group were significant.
Patellar Cartilage Thickness

MRI image of femorotibial joint depicts Patellar Cartilage Thickness of a 47 year old, female with primary knee OA (K-L grade II) before and after 180 days of ApresFlex supplementation.
Medial Tibia, Lateral Tibia, and Femoral Condyle Cartilage Thickness

MRI image of femorotibial joint depicts the joint space of a 47 year old, female with primary knee OA (K-L grade II) before and after 180 days of ApresFlex supplementation.
Joint Space

MRI image of femorotibial joint depicts the joint space of a 47 year old, female with primary knee OA (K-L grade II) before and after 180 days of ApresFlex supplementation.
CLINICAL HIGHLIGHTS
Clinical Study: Long-term study shows increasing joint comfort and cartilage preservation
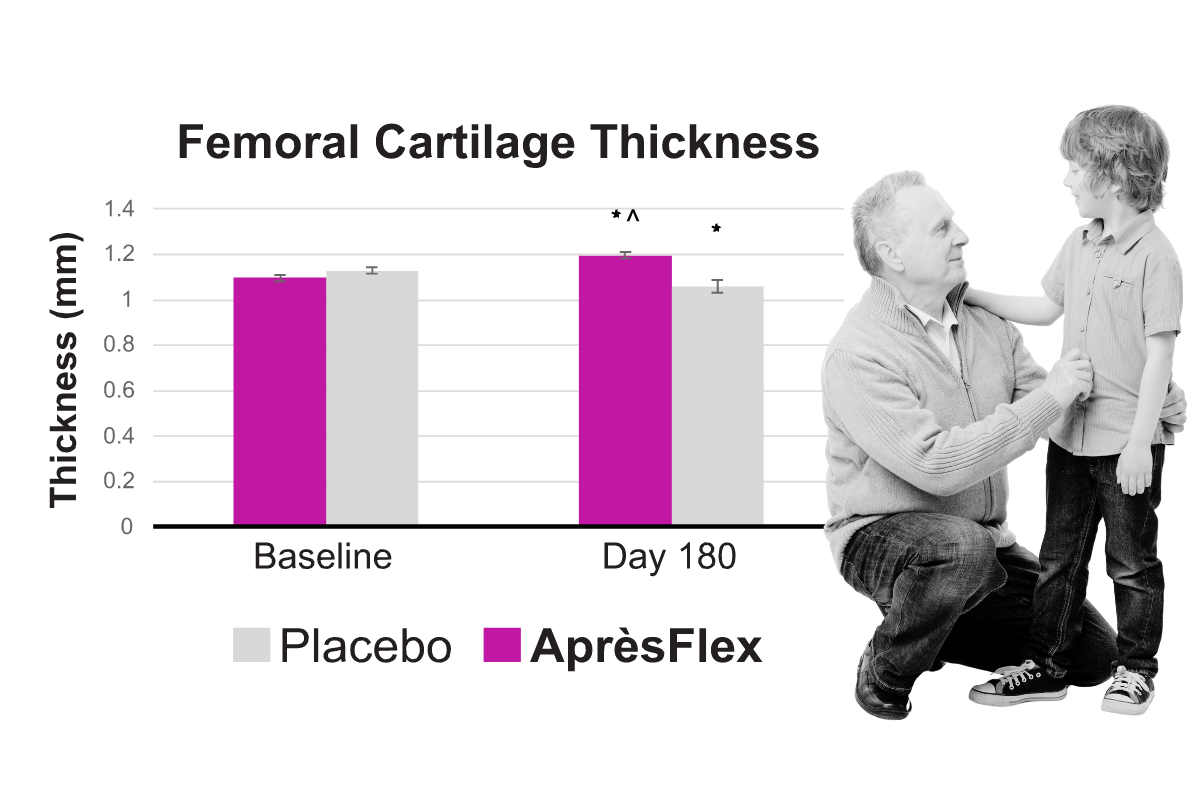
Eighty normal-to-overweight women and men (age 40-75) received either 100 mg/day of AprèsFlex or a matching placebo for six months. Subjects taking AprèsFlex experienced steady improvement in joint comfort and function with up to a 70% reduction in pain and a 72% reduction in stiffness by the end of the study. MRI assessments of joint space in the knee showed AprèsFlex limited joint space narrowing. MRIs of femoral, patellar, lateral tibial, and medial tibial cartilage thickness showed decreases in the placebo group over six months, whereas cartilage thickness was maintained and even slightly improved in the AprèsFlex group at 180 days.

Clinical Study: AprèsFlex improves joint comfort and function at 5 days
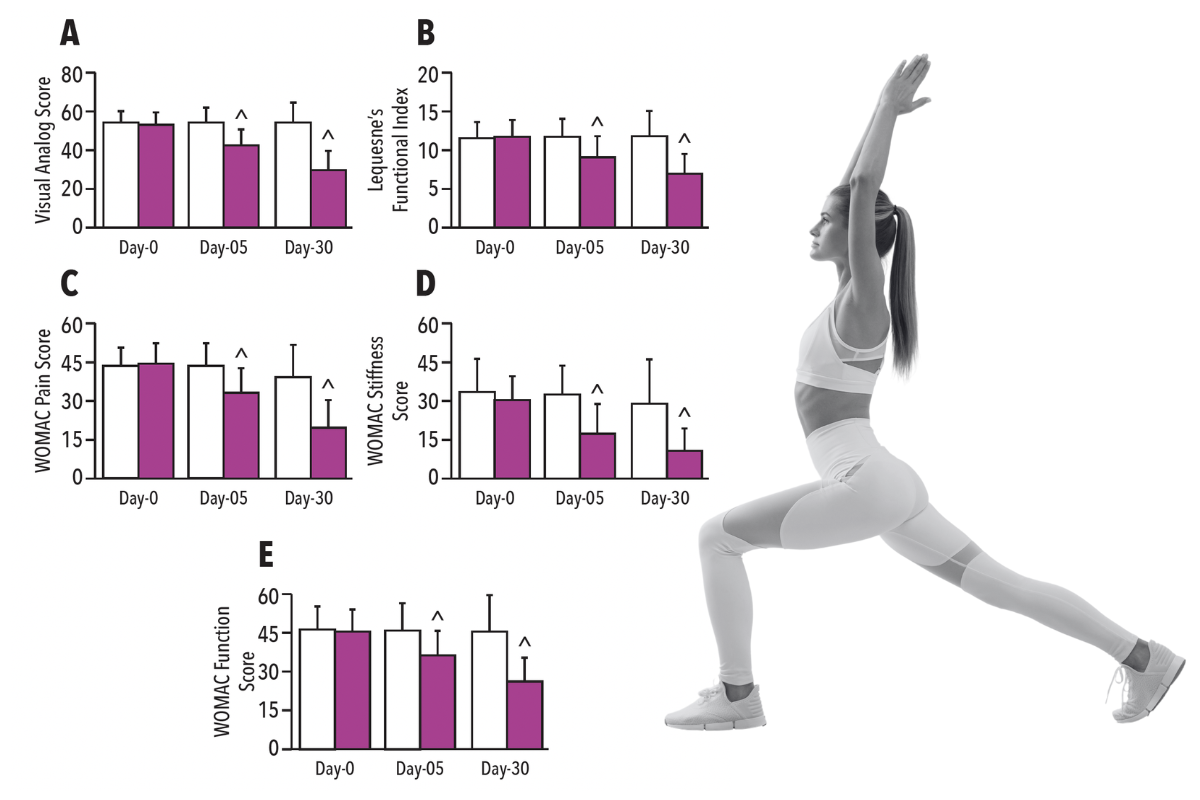
AprèsFlex was found to: provide significant reduction (p<0.05) in all pain scores at five days and to provide significant improvement(p<0.05) in all function scores at five days compared to placebo.
* Karlapudi V, Sunkara KB, Konda PR, Sarma KV, Rokkam MP. Efficacy and Safety of Aflapin®, a Novel Boswellia Serrata Extract, in the Treatment of Osteoarthritis of the Knee: A Short-Term 30-Day Randomized, Double-Blind, Placebo-Controlled Clinical Study. J Am Nutr Assoc. 2023;42(2):159-168. doi:10.1080/07315724.2021.2014370

Clinical Study: AprèsFlex® Improves Joint Comfort Starting at 5 Days

A 30-day, double-blind, randomized, placebo-controlled study was conducted to validate the efficacy of AprèsFlex® in the management of clinical symptoms of osteoarthritis (OA) of the knee Sixty eligible OA subjects selected through screening were included in the study. The subjects received either 100 mg (n=30) of Aflapin(®) or placebo (n=30) daily for 30 days. Each subject was evaluated for pain and physical functions by using the standard tools. AprèsFlex provided significant improvements in pain score and functional ability in as early as 5 days of treatment.
Vishal AA, Mishra A, Raychaudhuri SP. Int J Med Sci. 2011;8(7):615-622. doi:10.7150/ijms.8.615

Clinical Study: Groundbreaking MRI Analysis shows cartilage protection
Eighty women and men received either 100 mg/day of AprèsFlex or a matching placebo for six months. Subjects taking AprèsFlex showed limited joint space narrowing. MRIs of femoral, patellar, lateral tibial, and medial tibial cartilage thickness showed decreases in the placebo group over six months, whereas cartilage thickness was maintained and even slightly improved in the AprèsFlex group at 180 days. (Kumar, et. al. 2024)
Kumar, et. al.,A Standardized Boswellia serrata Extract Improves Knee Joint Function and Cartilage Morphology in Human Volunteers with Mild to Moderate Osteoarthritis in a Randomized Placebo-Controlled Study, Journal of the American Nutrition Association, DOI: 10.1080/27697061.2024.2438894

MARKET OPPORTUNITIES
Bone, Joint & Muscle Health
With AprèsFlex, you can make any joint health product work faster and better starting at 5 days – with a low dose.
Healthy Aging
Today’s seniors travel more, play more and do more than ever. AprèsFlex can help them get moving and keep moving.
Active/Sports Nutrition
Inflammation from exercise has similarities to that which occurs with the aging process.
Weight Management
Mobility and freedom from discomfort are critical to the kind of exercise we need to maintain a healthy weight.
ORIGIN STORY
It Starts with Boswellia
Boswellia serrata, native to India, is one of 19 species of the genus Boswellia. PLT Boswellia-based ingredients are sourced only from select regions of India.

A Storied Past
The plant resin of the Boswellia tree provides a commercial oil known as frankincense, which has a “woody, spicy and haunting smell.” It is possibly best known through the biblical story of the Three Wise Men, who delivered gifts of gold, frankincense, and myrrh.

Careful Harvesting
The harvest of Boswellia oleo gum resin occurs through “wounding and healing” of the tree, termed “tapping,” shallow debarking the tree to expose resin glands and stimulate resin production. Harvest is stopped from May through October or November in order to allow a period for tree recovery.

Exacting Analytics
Boswellia serrata oleo gum resin extracts are evaluated for unique characteristic features. Laila Nutraceuticals has developed an identification method using fingerprint profiles and high-performance thin-layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC).

State-of-the-Art Processing
Laila Nutraceuticals facilities maintain third-party Good Manufacturing Practices (GMP) certification from NSF International. The NSF certification includes compliance to the U.S. FDA Food Safety Modernization Act (FSMA) and cGMP 21 CFR 117 + 21 CFR 111.

QUALITY

From Harvest to Finished Ingredient
The starting point for 5-LOXIN – Boswellia serrata – is a plant that produces Indian frankincense. It is also known as Indian olibanum, Salai guggul, and Sallaki in Sanskrit. The plant is native to much of India and the Punjab region that extends into Pakistan. In partnership with Laila, PLT is committed to the safety and fair treatment of the people who harvest 5-LOXIN. We want to support the long-term health and well-being of the tribal culture and the natural environment upon which it relies.
Every batch of the 5-LOXIN harvest is assigned a specific batch number, and each batch of finished product is traced to a specific region and set of trees. 5-LOXIN is tested and must comply with predefined specifications for different parameters at different stages of product manufacturing – from raw materials to finished product. Quality is monitored by a multi-disciplinary organization with dedicated departments for taxonomy (botanical Identification), raw material analysis, chemical analysis, microbiology, and more.
SUSTAINABILITY
Understanding the Difference Makes the Difference
During the fourth quarter of 2021, PLT Health Solutions announced the commencement of a third-party sustainability audit program on Boswellia serrata trees, which serve as the raw material source for some of our most important ingredients. The audit of this India resource was conducted by renowned botanical research consultancy Botanical Liaisons, LLC, and was undertaken to help the nutraceutical industry and non-governmental agencies around the world understand what we and our ingredient innovation partner Laila Nutraceuticals have known for many years: proper stewardship of natural resources and social responsibility toward the communities that support our work are both the right thing to do and good business.
The audit program was a step in PLT’s campaign to document the sustainability of the tree resource. PLT’s Chairman, Paul Flowerman, made field trips to the key production areas in 2018 and 2019 to observe and document the variety of tree populations, methods of natural and forced propagation and the status of the tribal groups involved in the care of the trees and resin production.


A third audit program was based on a broad range of environmental, cultural and economic parameters. In March 2022 Botanical Liaisons reported to PLT its preliminary conclusions, which included this statement: “It is clear from the information collected from the stakeholders that [sustainability] is supported. Boswellia serrata has several sustainability advantages that prevent over-harvesting of PLT-sourced Boswellia compared to other sources and species of Boswellia.” The report recommended a series of next steps to further evaluate and promote sustainability.
Subsequently, four important additional developments have added considerable strength to the Boswellia serrata sustainability story:
- Authoritative tree survey data from Madhya Pradesh quantified the robustness and sufficiency of the tree resource to provide present and future resin requirements.
- CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) determined during its November 2022 meeting (COP 19 in Panama City) that no Boswellia species would get added to the CITES Appendix lists. The health of Boswellia serrata, the only species of commercial importance for PLT, was documented, including information about the replenishment of the resource.
- AHPA (American Herbal Products Association) convened an expert panel, which has drafted a brochure entitled, “Good Stewardship Harvesting of Boswellia (Boswellia Serrata).” This is a definitive presentation of current best practices.
- An important study modeling the impact of significant climate change by 2050 on Boswellia serrata resources predicted that almost all of Madhya Pradesh, the source of 90+% of commercial resin, will remain conducive for a healthy tree population.
These initiatives represent the type of work we do with our PLT360™ initiative. Introduced in 2015, our PLT360 initiative is a business-wide commitment by PLT Health Solutions to develop ingredients that our customers can be confident and proud to supply to their own customers – knowing that these ingredients are safe, of high quality, efficacious and harvested and manufactured sustainably. PLT360 examines every business decision and business process we undertake to be transparent with our operations, build trust with the health & well-being community and, together with them, support healthier, happier lives for the consumers we serve.
INSIGHTS
Flexibility is Freedom
When we play a round of golf, spend an afternoon on our knees in the garden or just take a long walk, we celebrate our freedom.
AprèsFlex has been giving people back their freedom for almost a decade by delivering joint comfort and flexibility from a 100 mg dose that starts to work in only 5 days.
At PLT, we are working on the concept of Mobility Solutions – which includes benefits for joints, muscle and cartilage. We’re thinking about athletes who want to perform better and recover faster. We’re thinking about people with physically demanding jobs who don’t want to or can’t take a day off. We’re thinking about anyone who has an active lifestyle and wants to embrace it despite the rigors. And yes, we’re thinking about the global aging population.
PLT Health Solutions’ leading portfolio of mobility support ingredients – based on more than a decade of ongoing research – has been developed to help you deliver innovative products that can capture the attention and the trust of consumers. All our ingredients are backed by multiple clinical studies – and offer rapid improvements from a low dose – in just about any delivery system you can imagine.
Around the world, people have turned to AprèsFlex more than a billion times as a drug-free approach to joint comfort. They trust the science. Trust the quality. And trust the experience.
![]()

“We worship and protect these trees and this tradition is also passed on from generation to generation.”
Collector in Madhya Pradesh
RESOURCES

AprèsFlex® 5-Day Joint Support Has Been Clinically Demonstrated to Improve Joint Comfort, Flexibility and Function as well as to Provide Ground-Breaking Cartilage Preservation and Protection


Systematic Review and Sub-group Meta-analysis Reviewed Nine Ingredients for Efficacy in Pain, Stiffness and Functionality
Discover new mobility solutions for all demographics with clinically supported botanical ingredients. Watch the webinar for insights and a groundbreaking clinical study.






Deliver Enhanced Joint Comfort Your Customers Can Feel
PLT has a broad range of joint health solutions that can work in almost any type of product you’re making.
- Expertise
- Ingredient Solutions
- All
- Animal Health & Wellness
- Beauty from Within
- Joint & Bone
- Cardiovascular
- Cognitive Performance
- Energy
- Functional Foods & Beverages
- Healthy Aging & Longevity
- Hydration+
- Immune & Respiratory Health
- Men’s Health
- Muscle Health
- Pain & Mobility
- Plant-Based Nutrition
- Sexual Health
- Sleep
- Sports & Active Nutrition
- Stress & Mood
- Weight Management
- Women’s Health
- Applications
- Resources
- PLT People & Planet
- About
These products are not intended to diagnose, treat, cure or prevent disease. This website is for informational purposes only.



















