-
Ingredient SolutionsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ApplicationsQuickly narrow your search. Browse products in our sortable catalog. View Catalog
-
ResourcesRecently Posted
-
PLT People & Planet
-
About
Our international network, passionate team of experts and extensive industry knowledge is what sets us apart.
 Seth FlowermanCEO
Seth FlowermanCEO
Zembrin®

OVERVIEW
Zembrin can help give people power over their lives by introducing a sense of calm serenity or alert focus. Clinically-studied, it has been shown to start working in as little as 2 hours and is safe enough to be used every day.

25mg
Clinical studies have shown that Zembrin delivers its benefits at a very low dose.

2 Hours
Zembrin is experiential. Studies have shown that it starts delivering in as little as 2 hours from use.

1 Billion
Number of times Zembrin has been taken around the world to provide experiential stress relief.

001
Zembrin was awarded the first ever Export and Bioprospecting permit to be issued by the South African government - #001.
PLT and HG&H's Partnership at Work
PLT works with some of the most innovative, socially-committed companies in the world in bringing our solutions for health & wellness to market. HG&H Pharmaceuticals from South Africa is a unique company with a fascinating story to tell your customers.
FEATURES & BENEFITS

Experiential (feel the benefits)
Fast-acting
Low dose (25mg)
Clinically studied
Extensive safety testing
Seed-to-shelf quality control
Ethically sourced
CERTIFICATIONS
VEGAN
GLUTEN-FREE
NON-GMO
HALAL
KOSHER
Helping people live their best lives
Zembrin is a novel, multi-patented, clinically studied extract of the South African succulent plant Sceletium tortuosum.
Zembrin is not an antidepressant, but it helps with calmness and serenity. It’s not a stimulant, but it helps with alertness and focus. It doesn’t cause drowsiness or sedation. It has no harmful side effects and does not present addiction potential – unlike many pharmaceuticals that may offer the same benefits.
Sceletium tortuosum
Sceletium tortuosum is a succulent plant in the family Aizoaceae, native to the Cape Provinces of South Africa.

An Ancient Helper
Sceletium has been used as a mood enhancer and stress reliever by the Khoi-San of South Africa, with a culture that stretches back over 20,000 years.
Rhodiola rosea
Rhodiola rosea is a plant native to remote Arctic climates in Asia, Europe and North America.

Exciting Phytochemistry
The root of the plant contains around 140 chemical compounds including phenols, rosavin, rosarin, salidroside and more.
Detail 1
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.

Detail 2
Kale chips cold-pressed put a bird on it mumblecore kogi brooklyn farm-to-table blue bottle yuccie authentic kombucha migas. Literally tilde tacos paleo.
RESEARCH
One of the industry’s most advanced science programs
Zembrin has been the subject of one of the most extensive and forward-thinking clinical science programs in the nutraceutical industry.
Zembrin’s clinical program has traveled the journey from mechanistic understanding of Zembrin’s function to relevant clinical endpoints in humans, supporting consumer communications about improved well-being. The present indications for Zembrin, as a dietary supplement are to elevate mood, relieve stress and improve cognition (focus). Adjectives like “anti-stress, calmness, focused, serenity, motivated, energy, improves sleep” are often used to describe the experiential effects of Zembrin.
Human clinical research
fMRI study: Anxiety is characterized by hyper-responsivity to mild threats. In 2013, investigators from the University of Cape Town in South Africa, led by Dr. David Terburg, tested the effects of Zembrin in a pharmacologic study with corroboration by Functional Magnetic Resonance Imaging (fMRI)2. The focus was to detect anxiety-related activity in the amygdala and its connected neurocircuitry. In a double-blind, placebo-controlled, cross-over design, sixteen (n=16) healthy participants were scanned during performance in a perceptual-load and an emotion-matching task. Amygdala reactivity to fearful faces under low perceptual load conditions was attenuated after a single 25 mg dose of Zembrin. Follow-up connectivity analysis on the emotion matching task showed that amygdala–hypothalamus coupling was also reduced. These results demonstrated, for the first time, the effects of S. tortuosum on the threat circuitry of the human brain. Zembrin was shown to attenuate reactivity within the amygdala, the part of the brain that responds to threats. It provides supporting evidence that the dual 5-HT reuptake inhibition and PDE4 inhibition of this extract might have anxiolytic potential by attenuating subcortical threat responsivity.
2 Terburg D, Syal S, Rosenberger LA, et al. Acute effects of Sceletium tortuosum (Zembrin), a dual 5-HT reuptake and PDE4 inhibitor, in the human amygdala and its connection to the hypothalamus. Neuropsychopharmacology. 2013;38(13):2708-2716. doi:10.1038/npp.2013.183
Cognition study: Converging evidence suggests that PDE-4 (phosphodiesterase subtype 4) plays a crucial role in regulating cognition via the PDE-4-cyclic AMP (cAMP) cascade signaling involving phosphorylated cAMP Response Element Binding Protein (CREB). To examine the neurocognitive effects of Zembrin and assess its safety and tolerability in cognitively healthy control subjects, researchers conducted a randomized, double-blind placebo-controlled crossover, proof-of-concept study of 21 healthy women and men (mean age 54.6 years.)3 Subjects received either 25 mg of Zembrin or matching placebo capsule once daily for 3 weeks. A battery of neuropsychological tests was used to assess the impact: CNS Vital Signs and Hamilton depression rating scale (HAM-D) at baseline and regular intervals and monitored side effects with treatment-emergent adverse events scale.
The CNS VitalSign® battery of tests assesses mood (i.e., fatigue, relaxed, tired, tense, upset, worried, alert, calm, hunger, thirst, anxious, stressed, happy) using visual analogues scales (VAS). It assesses cognition through computerized testing (stress through multitasking cognitive stressor FRAMEWORK; Cognitive tasks of attention, memory, and executive function). It assesses psychomotor function using biomarkers (blood pressure, cortisol, cortisol at bedtime) and a diary to track sleep quality, appetite, and adverse events. Secondary outcome measures were mood, assessed by the Hamilton Depression Rating Scale (HAM-D), safety, and tolerability.
Results: Zembrin significantly improved key cognitive domains, specifically cognitive set flexibility (𝑃 < 0.032) and executive function (𝑃 < 0.022), compared with the placebo group. Cognitive flexibility is the ability to shift attention between two or more tasks and the ability to adapt to rapidly changing directions and intelligently utilize information. It is necessary for decision-making, impulse control, and strategy formation. Executive function refers to exercising initiative, proper judgment, discipline, and the ability to operate in favor of an abstract reward; spontaneous ability to take action, and goal-directed behavior.
Positive changes in mood and sleep were also found, although changes in the HAM-D scores did not reach statistical significance, an expected outcome in a healthy, non-depressed population. Zembrin was well-tolerated. The authors concluded that the cognitive-enhancing effects of Zembrin were likely due to its impact on the PDE-4-cAMP-CREB cascade, offering potential applicability for support of cognitive function in older individuals.
3 Chiu S, Gericke N, Farina-Woodbury M, et al. Proof-of-Concept Randomized Controlled Study of Cognition Effects of the Proprietary Extract Sceletium tortuosum (Zembrin) Targeting Phosphodiesterase-4 in Cognitively Healthy Subjects: Implications for Alzheimer's Dementia. Evid Based Complement Alternat Med. 2014;2014:682014. doi:10.1155/2014/682014
Single-dose EEG study: This study aimed to characterize the psychophysiological effects of a single dose of Zembrin of either 25 mg or 50 mg compared to placebo.4 The study employed a novel methodology called “EnkephaloVision”, developed by German brain researcher, Wilfried Dimpfel. The technology uses quantitative electroencephalogram (qEEG) software combined with eye-tracking, allowing for rapid detection and sensitive analysis of spectral EEG changes during cognitive and emotional challenges.
Sixty subjects were given a series of mental tasks and exposed to emotional audio-visual clips before and 2 hours after intake of Zembrin or placebo. The tests comprised five cognitive challenges (Stroop-Test, Brain Teaser, Memory-Test, Picture Comparison, CPT-Test) and four emotional challenges (Emotion Pictures, Animal Videos, and Horror Videos).
In comparison to the placebo, Zembrin induced enhanced increases in power of frontal delta, theta, alpha1, and alpha2 brain wave frequencies during several tasks. Increases in these frequencies in the frontal brain have been related to attention and memory. These results may represent a positive dose-dependent action of Zembrin on cognitive and emotional processes in the brain.
Six-week EEG study: The following year, the same research group did a follow-up study to measure the effects of 25 or 50 mg of Zembrin taken daily for 6 weeks compared to placebo.5 Sixty healthy male (n = 32) and female (n = 28) right-handed subjects between 50 and 80 years of age took part in the study. The EEG was recorded from 17 surface electrodes before and 1 hour after intake. Six cognitive tests were performed: d2-test, memory test, calculation performance test, reaction time test, number identifying test, and number connection test. Three questionnaires were included: Profile of Mood States, Hamilton Anxiety Rating Scale and a sleep questionnaire.
Quantitative EEG showed increases in delta activity during the performance of the d2-test, the number identification and number connection test in the fronto-temporal brain region. Higher theta activity was seen during relaxation and performance of the d2-test after intake of 50 mg of Zembrin. Increases of alpha1 spectral power were seen in the relaxed state. With respect to alpha2 spectral power, larger increases were observed in the centro-occipital region. Statistically significant improvement during performance of the arithmetic calculation test and number connection test was documented. The Hamilton anxiety score (HAM-A) revealed a statistically significant decrease (p = 0.03) after six weeks. The results indicate that in healthy people, Zembrin improves some aspects of cognitive function, decreases anxiety, and may enhance mood.
Single-dose anxiety study: In a two-part study, researchers used a double-blind, placebo-controlled, between-subject experimental design to investigate the effects of a single dose of Zembrin (25 mg) on induced stress/anxiety response in 20 young, healthy volunteers.6 To elicit feelings of stress/anxiety, participants completed 20 minutes of the multitasking framework in Part 1; and a 5-minute simulated public speaking task in Part 2. Part 1 measured subjective experiences of mood at baseline, pre-stress induction, and post-stress induction. Part 2 measured subjective experiences of anxiety and physiological indicators of stress (heart rate and galvanic skin response) at baseline, pre-stress induction, during stress induction, and post-stress induction.
Results: No treatment effect was observed in Part 1; however, Part 2 revealed subjective anxiety levels to be significantly lower in the Zembrin group at the pre-stress induction point and a significant interaction between treatment and time on heart rate. Taken together, results indicate that a single dose of Zembrin can ameliorate induced stress/anxiety response in healthy volunteers, such as occurs before public speaking. The authors opine that the likely reason Part 1 failed to show a treatment effect is that the "stressor was too 'mild' to allow a treatment effect to be observed in those subjective self-report measures." It is also likely that daily administration, as opposed to a single dose, would produce a more pronounced effect.
4 Dimpfel W, Gericke N, Suliman S, Dipah GNC. Psychophysiological Effects of Zembrin® Using Quantitative EEG Source Density in Combination with Eye-Tracking in 60 Healthy Subjects. A Double-Blind, Randomized, Placebo-Controlled, 3-Armed Study with Parallel Design. Neurosci Med. 2016;7:114-32. doi:10.4236/nm.2016.73013
5 Dimpfel W, Gericke N, Suliman S, Dipah GNC. Effect of Zembrin® on Brain Electrical Activity
in 60 Older Subjects after 6 Weeks of Daily Intake. A Prospective, Randomized, Double-Blind, Placebo-Controlled, 3-Armed Study in a Parallel Design. World J Neurosci. 2017;7:140-71.
6 Reay J, Wetherell MA, Morton E, Lillis J, Badmaev V. Sceletium tortuosum (Zembrin®) ameliorates experimentally induced anxiety in healthy volunteers [published online ahead of print, 2020 Aug 6]. Hum Psychopharmacol. 2020;e2753. doi:10.1002/hup.2753
CLINICAL HIGHLIGHTS
Clinical Study: fMRI study shows Zembrin reduced anxiety response within 2 hours
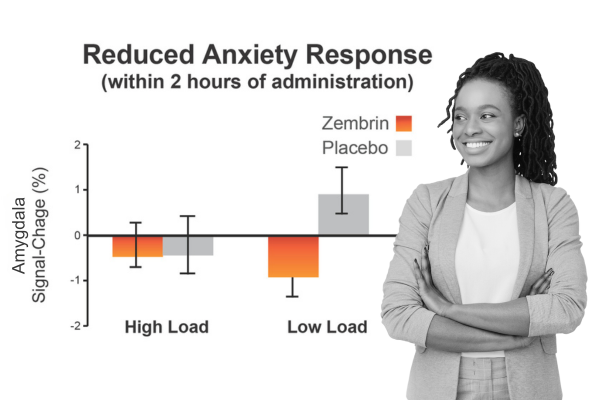
In a randomized, double-blind, placebo-controlled study with 16 healthy subjects, researchers used functional magnetic resonance imaging (fMRI) to detect anxiety-related activity in the amygdala after a single 25-mg dose of Zembrin. This was the first demonstration of effects of S. tortuosum on the threat circuitry of the brain supporting the presumptive MOA – that dual 5-HT reuptake inhibition and PDE4 inhibition calm stress reactions by attenuating subcortical threat responsivity.
Terburg D et al. Neuropsychopharmacol. 2013;38(13):2708-16.

Clinical Study: Zembrin significantly improves key cognitive domains
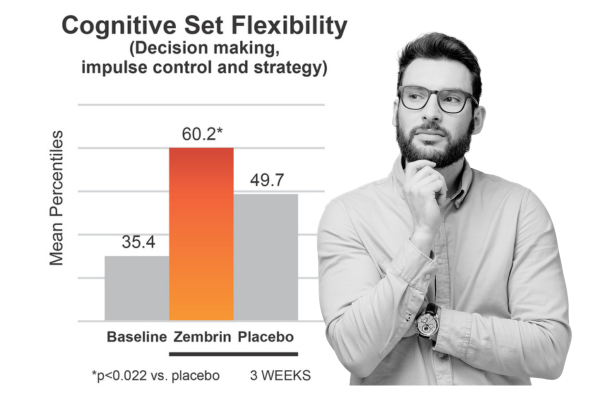
Subjects in a crossover study took Zembrin or placebo for 3 weeks followed by a 3 week washout period and then the opposite treatment. Mood and cognitive psychomotor function were assessed. Zembrin significantly improved key cognitive domains, specifically cognitive set flexibility (𝑃 < 0.032) and executive function (𝑃 < 0.022), compared with the placebo group.

Clinical Study: Zembrin improved stress and mood scores, even in healthy subjects
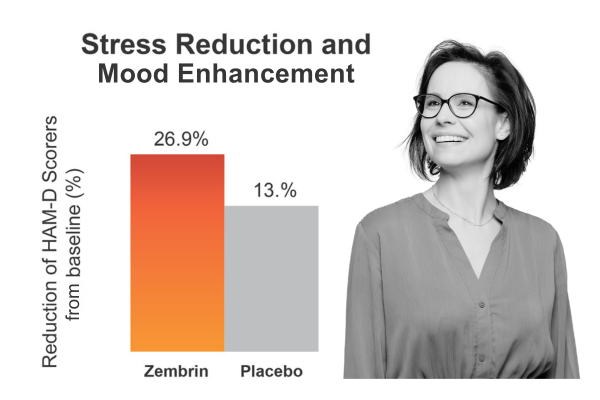
25 mg/d Zembrin or matching placebo was administered to subjects for 3 weeks Positive changes in mood and sleep were found, although changes in the HAM-D scores did not reach statistical significance, an expected outcome in a healthy, non-depressed population.
Chiu S et al.. Evid Based Complement Alternat Med. 2014;2014:682014.

Clinical Study: Zembrin demonstrates Anxiolytic Properties

Two studies investigated the effects of a single dose of Sceletium tortuosum (25 mg, Zembrin® ) on laboratory stress/anxiety responding in 20 young healthy volunteers. To elicit feelings of stress/anxiety, participants completed 20 min of the multitasking framework in study 1 and a 5-min simulated public speaking task in study 2. Study 2 measured subjective experiences of anxiety and physiological indicators of stress (heart rate [HR] and galvanic skin response) at baseline, prestress induction, during stress induction, and post stress induction. Study 2 revealed subjective anxiety levels to be significantly lower in the Zembrin® group at the prestress induction point .
Reay J, et al... Sceletium tortuosum (Zembrin® ) ameliorates experimentally induced anxiety in healthy volunteers. Hum Psychopharmacol. 2020;35(6):1-7. doi:10.1002/hup.2753

MARKET OPPORTUNITIES
Active/Sports Nutrition
Training requires focus and overcoming the stresses of everyday life. That’s where adding Zembrin to your sports formulation can help.
COGNITIVE / MOOD SUPPORT
Zembrin has been clinically demonstrated to improve cognitive flexibility and executive function. Users “feel” its benefits.
E-SPORTS
Enhanced cognitive function is one of the most sought-after benefits by gamers who take supplements.
Weight Management
Zembrin can help make the long road to weight loss success seem just a little less long.
APPLICATIONS

BEVERAGES

CAPSULES

CHEWS

GUMMIES
EFFERVESCENTS

POWDERS

RTDS/SHOTS

SOFT GELS

STICK PACKS

TABLETS
ORIGIN STORY
Ancient knowledge. Modern science.
When ingredient innovator HG&H Pharmaceuticals set out to develop Zembrin, they were determined to formally acknowledge and reward the contribution of the Khoi-San people who had discovered and used the South African plant Sceletium for millennia.

The First Export and Bioprospecting Permit
Zembrin was awarded the first ever Export and Bioprospecting permit from the South African government - #001 - which was issued compliant with the National Environmental Management; Biodiversity Act.

The South African San Council
Zembrin is endorsed by the South African San Council, with the exclusive use of their trademark for a Sceletium product. The San Council shares in the proceeds from the sale of every bottle of Zembrin.

An Ancient Story
When your customers purchase a product containing Zembrin, they become part of a story that stretches back thousands of years and supports the people who brought knowledge of this ingredient to the world.

QUALITY
Modern agronomy improves traditional medicine
Sceletium tortuosum is a protected species and wild-harvesting is not sustainable for commercial products. Zembrin innovator, HG&H Pharmaceuticals, employs a seed-to-shelf ingredient management program with its specially cultivated Sceletium tortuosum raw materials. The company is committed to using only cultivated plant material to avoid depleting threatened wild plant stocks and to ensure consistent product quality.
Each batch of Zembrin is traceable to an individual seed and plant.
At harvesting, the material is checked for quality and the Zembrin ‘fingerprint’ using innovative analytical technology.
Zembrin represents the application of cutting-edge neuro-scientific research and sustainable agricultural technology to a traditional plant to address the unmet need for a rapidly acting, safe, effective and experiential stress relieving ingredient.

INSIGHTS
Zembrin is Experiential
One of the most intriguing aspects of Zembrin is that it is experiential – users “feel” its benefits. It is also fast-acting. A published study that used functional Magnetic Resonance Imaging (fMRI) technology to examine the effects of short-term supplementation with Zembrin on the “threat circuitry” of the human brain showed statistically significant reductions in anxiety-related activity of the amygdala and its associated anxiety circuitry within 2 hours of administration.
Cognitive Support for Peak Performance
At PLT Health Solutions, we have been focusing on what we consider to be an underserved demographic – the 18-54 age range. Within this group, we see intense interest in issues such as ‘peak performance’, ‘well-being’ and ‘quality of life’. The market opportunity with this group is massive. Peak performers, including knowledge workers and students, make up 40% of the US population and is growing. Stress affects 75 million people in this group. And the impact of cognitive performance on other aspects of life is becoming better understood by this demographic– as evidenced by the introduction of ingredients to improve mental performance in exercise via sports nutrition products as well as easier weight management.

“South Africa believes that meaningful partnership and collaboration between the traditional knowledge holders and scientific community or research institution is critical for ABS success.”
The Honourable Edna Molewa, South African Minister of Environmental Affairs
RESOURCES



Explore the latest in cognitive support for well-being with PLT's ingredient platform. In this webinar, you'll discover the science behind cognitive ingredients and how to incorporate them into formulations.











Help your customers live their best lives.
PLT has a broad range of cognitive health support solutions with strong scientific support. Find out how we can help you put these ingredients to work in your products.
- Expertise
- Ingredient Solutions
- All
- Animal Health & Wellness
- Beauty from Within
- Joint & Bone
- Cardiovascular
- Cognitive Performance
- Energy
- Functional Foods & Beverages
- Healthy Aging & Longevity
- Hydration+
- Immune & Respiratory Health
- Men’s Health
- Muscle Health
- Pain & Mobility
- Plant-Based Nutrition
- Sexual Health
- Sleep
- Sports & Active Nutrition
- Stress & Mood
- Weight Management
- Women’s Health
- Applications
- Resources
- PLT People & Planet
- About
These products are not intended to diagnose, treat, cure or prevent disease. This website is for informational purposes only.














